Posted
1st September 2020
Research
Sourcing up-to-date reliable information on COVID-19 management and treatment can be difficult. There are so many different sources of information, from peer-reviewed journals, mainstream media, social media, peers, media statements and ‘preprints’ – or non-peer reviewed journal articles.
What makes this even more complicated is the potential bias associated with some of these sources of information. The key question then springs to mind. How can I find reliable information? Information where all the work has been done – assessing bias, quality of evidence, synthesising this with existing literature and consider the practical and clinical implications of recommendations. This is where a COVID-19 Clinical Evidence Taskforce comes in very handy.
The Taskforce is a diverse coalition of peak health bodies that focus on clinical care of Australians with COVID-19. At the time of writing, the number of peak bodies involved stands at 28 and includes the Australasian College for Infection Prevention and Control (ACIPC), Australian and New Zealand College of Anaesthetists (ANZCA), Australian and New Zealand Intensive Care Society (ANZICS), Royal Australian College of General Practitioners (RACGP) and many more found here. Despite being led in Australia, the evidence is applicable internationally.
A first of its kind, the national COVID-19 clinical evidence Taskforce provides a clear and consistent voice of cross-disciplinary consensus on the clinical care of patients with COVID-19. The team work around the clock to analyse global research findings, evaluate the evidence, collate and prioritise clinicians’ questions and update COVID-19 clinical guidelines and clinical flowcharts. Traditionally, clinical guidelines are updated every few years, but Taskforce have developed ‘living’ guidelines, updated each and every week.
The evidence surveillance system used tracks and identifies relevant studies as results are allowing teams to identify and summarise global COVID-19 research findings. Findings are synthesised and reviewed, by feed, into 15 expert guideline panels where recommendations are formed. Recommendations are then reviewed by the Leadership Group each week, which comprises of the 29 leading health organisations covering primary, acute and critical care settings around Australia. Where full consensus has been met from all organisations, recommendations are then published.
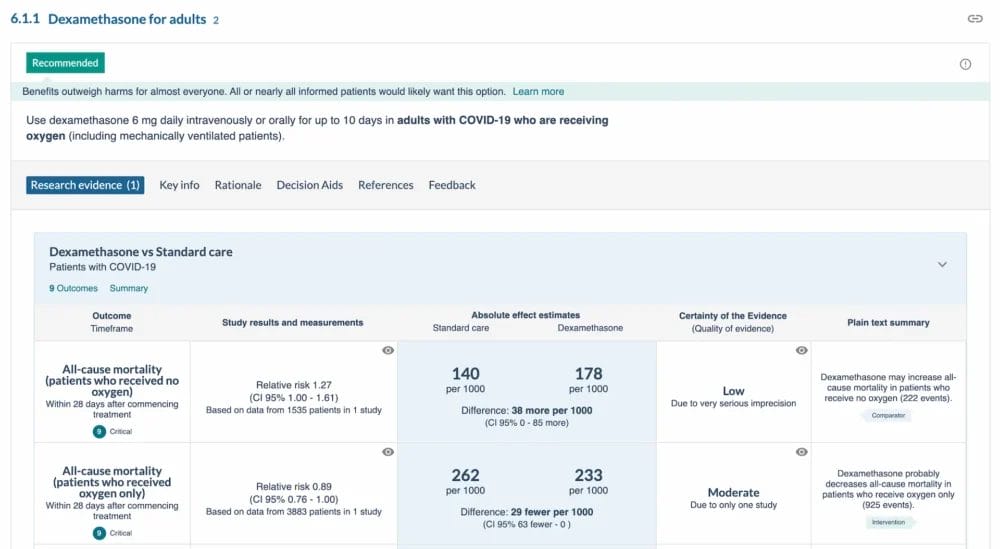
Recommendations are presented as a flowchart or in a living guideline. Living guidelines contain detailed information regarding the reviews undertaken including certainty of evidence, evidence summaries, forest plots, rationale and more. All information and data is transparently reported. An example of one recommendation is below. The Taskforce include pre-prints in the evidence reviews, providing full data is available. Certainty of evidence and the data is reviewed again, once the final publication is published.
Such an approach does require considerable resources, as well as voluntary work. The approach used in a world first and in times of a pandemic, having clear transparent information is critical.
SHARE THIS ARTICLE
Tags
Latest News
Introducing HEXI HUB: A seamless transition in our product line
We’re pleased to announce an update to our product offering…
Innovative solutions for tackling Carbapenemase-producing Enterobacteriaceae (CPE) at King’s College Hospitals
King’s College Hospital NHS Foundation Trust, one of London’s largest…
Gloves Off: reducing unnecessary plastic waste during environmental cleaning and disinfection
In this blog, Dr Phil Norville discusses the momentum-gaining ‘Gloves…
Gloves Off: Navigating SDS sheets and skin safety claims in environmental decontamination products
In this blog, James Clarke (Head of R&D, Science &…